Abstract
Introduction: T-cell prolymphocytic leukemia (T-PLL) is a rare disease with unfavorable prognosis. Patients who are fit at presentation are treated with alemtuzumab and allogenic stem cell transplantation (alloSCT), whereas patients with poor performance status or concurrent malignancies receive alemtuzumab only or are not treated. Patients who are not candidate for alloSCT in first-line, are in a palliative trajectory. Evaluation of the impact of treatment regimens on outcome among T-PLL patients is needed. However, due to its rarity and aggressive disease course, randomized clinical trials are not available. Therefore, population-based data is needed to evaluate outcome among T-PLL patients.
Aim: This nationwide population-based cohort study evaluates first-line treatment and outcome of patients with T-PLL in the Netherlands.
Methods: All newly diagnosed T-PLL patients ≥18 years who were diagnosed in 2014-2020 were identified in the Netherlands Cancer Registry, with survival follow-up up through February 1, 2022. Baseline characteristics and primary treatment regimens were collected. Patients were categorized into three treatment groups, e.g. alemtuzumab, other, and no therapy, all in first-line. The primary endpoint was overall survival (OS), defined as the time from T-PLL diagnosis to all-cause-death.
Results: In total, 87 newly diagnosed T-PLL patients were identified, with a median age of 73 years (range 38-91 years) and of whom 43% was female. Of these patients, 33 (38%) received alemtuzumab in first-line, 15 (17%) received other treatment such as CHOP21 or fludarabine, and 39 (45%) did not receive treatment. Reasons for not receiving first-line therapy were divers, including poor functional status, comorbidities, and the patients' explicit wish not to be treated. For the 33 patients who received alemtuzumab, 4 received CHOP simultaneously and 14 patients underwent subsequent alloSCT. Moreover, 6 (18%) patients received 1-4 cycles alemtuzumab (due to progressive disease (n=3) or no response (n=3)), 7 (21%) 5-8 cycles, 19 (58%) more than 8 cycles and for 1 patient, the number of cycles was unknown. Patients who underwent alloSCT, received ≥5 cycles of alemtuzumab.
The best overall response rates (ORR; complete remission + partial remission) for patients who received alemtuzumab or other therapy were 64% and 13%, respectively (p<0.01). ORR was 16% for patients who received 1-4 cycles of alemtuzumab, 57% for patients who received 5-8 cycles, and 79% for patients who received more than 8 cycles (p=0.04).
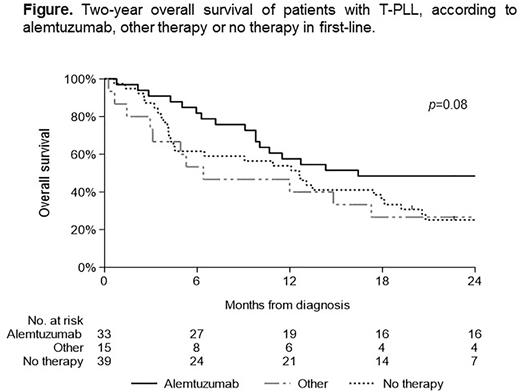
The median follow-up time for patients with alemtuzumab was 20.3 months (range; 0.8-75.5 months), for patients with other therapy 6.4 months (range; 0.3-71.5 months) and for patients without therapy 4.1 months (range; 0.6-66.2 months), of whom 7, 1, and 6 patients, respectively, were still alive on February 1, 2022. As expected, 2-year OS was highest for patients who received alemtuzumab, followed by patients treated with other and no therapy (50%, 27%, and 23%, respectively; p=0.08; Figure). Two-year OS was 17%, 57%, and 53% for patients who received 1-4 cycles of alemtuzumab, 5-8 cycles and more than 8 cycles, respectively (p<0.01). For patients who received alloSCT following alemtuzumab, 2-year OS was higher than for patients who received alemtuzumab without alloSCT (56% vs 28%; p=0.27).
Conclusion: In summary, for patients with ≥5 cycles of alemtuzumab and for patients who underwent alloSCT, 2-year survival is above 50%. Outcome remains very poor in T-PLL patients who receive no or less than 4 cycles of alemtuzumab. Therefore, new therapy strategies are urgently needed.
Disclosures
Levin:AbbVie, Roche and Janssen: Other: Travel expenses. Doorduijn:Eli Lilly/Loxo: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kater:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/genentech: Membership on an entity's Board of Directors or advisory committees; LAVA: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: pending; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Patents & Royalties: pending, Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal